Photosynthesis is the largest energy and materials conversion process, and provides the basis for survival and development of virtually all life forms on the earth.
To maintain a maximal efficiency of photosynthetic electron transport and to avoid photodamage when exposed to excess light, plants and green algae redistribute the excitation energy between the Photosystem I (PSI) and Photosystem II (PSII) by moving part of the PSII light-harvesting complex (LHCII) through state transition. In state 2, a part of LHCII moves to PSI, forming a PSI-LHCI-LHCII supercomplex.
The green alga Chlamydomonas reinhardtii (C. reinhardtii) exhibits state transition to a far larger extent than higher plants, however, the exact location and detailed association pattern of the LHCII trimers with the green algal PSI remain obscure due to the limited resolution.
A research team from the key laboratory of Photobiology, Institute of Botany, Chinese Academy of Science, and their collaborator from Zhejiang University solved the structure of PSI-LHCI-LHCII supercomplex from C. reinhardtii in state 2 using single particle cryo-EM at an overall resolution of 3.42 Å. This study is published in Nature communications on February 17th, 2021.
The structure reveals that the PSI-LHCI-LHCII of C. reinhardtii binds two LHCII trimers in addition to ten LHCI subunits. Two LHCII trimers located close to each other at the PsaO-PsaL-PsaH-Lhca2 side. One LHCII trimer (LHCII-1) attaches to the PSI core by PsaO, PsaH and PsaL subunits, whereas the other LHCII trimer (LHCII-2) attaches to the Lhca2 and LHCII-1 subunits.
The phosphorylation of the N-terminal Thr residue of the LHCII-1 trimer in the stromal side upon state 1 to state 2 transition induces extensive contacts of LHCII-1 with the PsaO/PsaH/PsaL subunits, which stabilize its binding to the PSI core.
Meanwhile, the present results also reveal the distribution of pigments as well as the plausible excitation energy transfer (EET) pathways in the Cr-PSI-LHCI-LHCII and demonstrate a highly efficient network of EETs involved in the state transition of the green alga C. reinhardtii.
“This is the first high-resolution structure of the PSI-LHCI-LHCII supercomplex from a green algae,” said Dr Han Guangye, one of the corresponding author from IBCAS. “This structure is very important because green algae grow under water or in soil and suffer different light conditions, which results in unique organization of this supercomplex in state 2.”
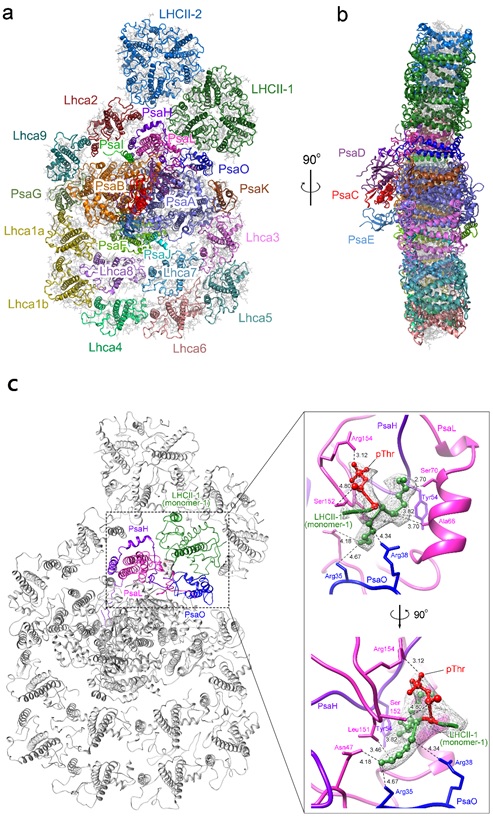
Over architecture of the PSI-LHCI-LHCII supercomplex of C. reinhardtii. Overall structure the Cr-PSI-LHCI-LHCII supercomplex viewed along the membrane normal from the stromal side (a) and along the membrane plane (b). (c) The structure of the phosphorylation site of LHCII and its interactions with the PSI core subunits of C. reinhardtii. (Image by IBCAS)
Article link: https://www.nature.com/articles/s41467-021-21362-6
Contact: Email: hanguangye@ibcas.ac.cn
Institute of Botany, Chinese Academy of Sciences

