‘Jin Yin Hua’, which has been widely used as a traditional Chinese herbal medicine for thousands of years, is derived from the flower buds of several Lonicera species. Among these species, Lonicera macranthoides Hand.-Mazz. mainly distributes in southern China and has long been used as ‘Jin Yin Hua’ in prescriptions. However, according to Chinese Pharmacopoeia 2005 edition, L. japonica Thunb. was recorded as the only natural plant source of ‘Jin Yin Hua’, while L. macranthoides was classified to the sources of ‘Shan Yin Hua’. The division of ‘Jin Yin Hua’ and ‘Shan Yin Hua’ resulted in a large decrease in demand for L. macranthoides atherbal medicine market and caused a great economic loss to the farmers. Hence, an argument arose that whether L. macranthoides can continue to be used as ‘Jin Yin Hua’. The debate focused on the content and biosynthesis of luteolin and/or luteolin-7-O-glucoside (Lu-7-Glc), the main efficacy components from flower buds of L. japonica, which have been linked to a range of potential health benefits in many reports and thought to be less abundant in the flower buds of L. macranthoides. Concerning this issue, Prof. WANG Liangsheng’s research group used L. japonica and L. macranthoides as plant materials and discussed the functional characterization of FNSII enzymes from the two Lonicera species, to reveal the reason for the low levels of flavones in the flower buds of L. macranthoides. They found that flavone synthase (FNS), the key enzyme responsible for flavone biosynthesis, were different between L. japonica and L. macranthoides. In the presence of NADPH, the recombinant cytochrome P450 proteins encoded by LjFNSII-1.1, LjFNSII-2.1, and LmFNSII-1.1 can convert eriodictyol, naringenin, and liquiritigenin to the corresponding flavones directly. The different catalytic properties between LjFNSII-2.1 and LjFNSII-1.1 were caused by a single amino acid substitution at position 242 (glutamic acid to lysine), where an acidic amino acid can contribute to the α -helix stability but weaken the catalytic efficacy. A methionine at position 206 and a leucine at position 381 contributed considerably to the high catalytic activity of LjFNSII-1.1. In addition, LjFNSII-1.1&2.1 and LmFNSII-1.1 also biosynthesize flavones that were further modified by O-glycosylation in transgenic tobacco. These findings suggested that the weak catalytic activity of LmFNSII-1.1 and the relatively low expression of LmFNSII-1.1 in flowers might be responsible for the low levels of flavone accumulation in flower buds of L. macranthoides. Unlike L. japonica, which blossoms sporadically, a wide range of varieties originated from L. macranthoides are covered with inflorescences and each inflorescence has many flowers that are simultaneously in bloom. These traits make L. macranthoides more suitable for convenient and efficient harvesting. The work suggests that the flexibility of plant metabolism allows the diversion of a substrate towards the biosynthesis of health-beneficial flavone derivatives. Considering the abundance of its flower buds, L. macranthoides has the prospect of becoming a good substitute for L. japonica if the elevated levels of Lu-7-Glc can be achieved through molecular breeding. This work has a profound significance for the quality improvement and innovation of new varieties of L. macranthoides. These results were published in Scientific Reports on January 12, 2016 (DOI: 10.1038/srep19245). PhD student WU Jie in Prof. WANG Liangsheng’s lab is the first author. This work is supported by the National Science and Technology Basic Project of China (Grant No. 2014FY110100). 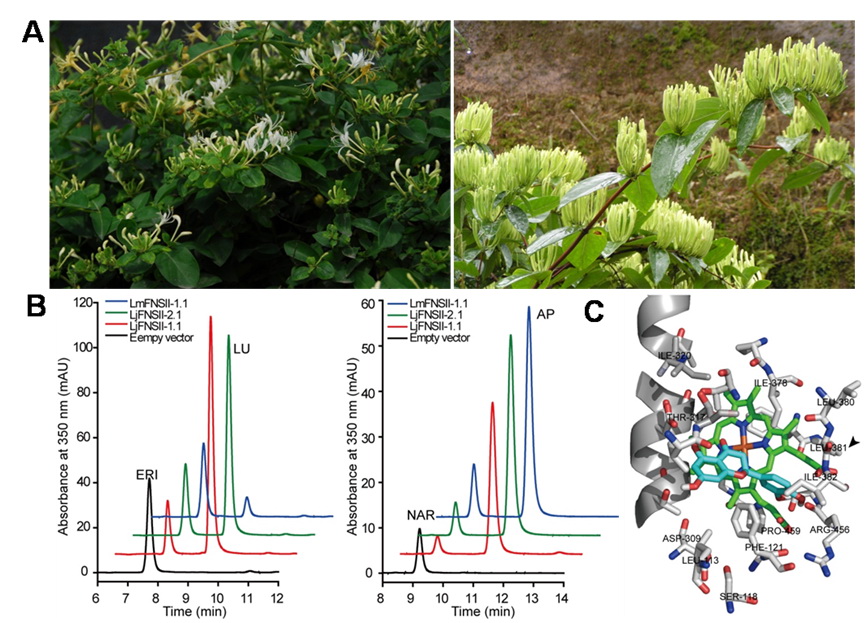
Figure (A) Lonicera japonica Thunb. (left) and L. macranthoides Hand.-Mazz.(right). (B) HPLC profiles of extracts from yeast cells expressingLjFNSII-1.1&2.1 and LmFNSII-1.1 show the direct conversion of eriodictyol (ERI) to luteolin (LU, left), and naringenin (NAR) to apigenin (AP, right), respectively. (C) Molecular model of the active site of LjFNSII-1.1.
Article link: http://www.nature.com/articles/srep19245
Contact information
Prof. WANG Liangsheng
Key Laboratory of Plant Resources/Beijing Botanical Garden
Institute of Botany, Chinese Academy of Sciences, Beijing 100093, China
E-mail: wanglsh@ibcas.ac.cn
‘Jin Yin Hua’, which has been widely used as a traditional Chinese herbal medicine for thousands of years, is derived from the flower buds of several Lonicera species. Among these species, Lonicera macranthoides Hand.-Mazz. mainly distributes in southern China and has long been used as ‘Jin Yin Hua’ in prescriptions. However, according to Chinese Pharmacopoeia 2005 edition, L. japonica Thunb. was recorded as the only natural plant source of ‘Jin Yin Hua’, while L. macranthoides was classified to the sources of ‘Shan Yin Hua’. The division of ‘Jin Yin Hua’ and ‘Shan Yin Hua’ resulted in a large decrease in demand for L. macranthoides atherbal medicine market and caused a great economic loss to the farmers. Hence, an argument arose that whether L. macranthoides can continue to be used as ‘Jin Yin Hua’. The debate focused on the content and biosynthesis of luteolin and/or luteolin-7-O-glucoside (Lu-7-Glc), the main efficacy components from flower buds of L. japonica, which have been linked to a range of potential health benefits in many reports and thought to be less abundant in the flower buds of L. macranthoides. Concerning this issue, Prof. WANG Liangsheng’s research group used L. japonica and L. macranthoides as plant materials and discussed the functional characterization of FNSII enzymes from the two Lonicera species, to reveal the reason for the low levels of flavones in the flower buds of L. macranthoides.
They found that flavone synthase (FNS), the key enzyme responsible for flavone biosynthesis, were different between L. japonica and L. macranthoides. In the presence of NADPH, the recombinant cytochrome P450 proteins encoded by LjFNSII-1.1, LjFNSII-2.1, and LmFNSII-1.1 can convert eriodictyol, naringenin, and liquiritigenin to the corresponding flavones directly. The different catalytic properties between LjFNSII-2.1 and LjFNSII-1.1 were caused by a single amino acid substitution at position 242 (glutamic acid to lysine), where an acidic amino acid can contribute to the α -helix stability but weaken the catalytic efficacy. A methionine at position 206 and a leucine at position 381 contributed considerably to the high catalytic activity of LjFNSII-1.1. In addition, LjFNSII-1.1&2.1 and LmFNSII-1.1 also biosynthesize flavones that were further modified by O-glycosylation in transgenic tobacco. These findings suggested that the weak catalytic activity of LmFNSII-1.1 and the relatively low expression of LmFNSII-1.1 in flowers might be responsible for the low levels of flavone accumulation in flower buds of L. macranthoides.
Unlike L. japonica, which blossoms sporadically, a wide range of varieties originated from L. macranthoides are covered with inflorescences and each inflorescence has many flowers that are simultaneously in bloom. These traits make L. macranthoides more suitable for convenient and efficient harvesting. The work suggests that the flexibility of plant metabolism allows the diversion of a substrate towards the biosynthesis of health-beneficial flavone derivatives. Considering the abundance of its flower buds, L. macranthoides has the prospect of becoming a good substitute for L. japonica if the elevated levels of Lu-7-Glc can be achieved through molecular breeding. This work has a profound significance for the quality improvement and innovation of new varieties of L. macranthoides.
These results were published in Scientific Reports on January 12, 2016 (DOI: 10.1038/srep19245). PhD student WU Jie in Prof. WANG Liangsheng’s lab is the first author. This work is supported by the National Science and Technology Basic Project of China (Grant No. 2014FY110100).

Figure (A) Lonicera japonica Thunb. (left) and L. macranthoides Hand.-Mazz.(right). (B) HPLC profiles of extracts from yeast cells expressingLjFNSII-1.1&2.1 and LmFNSII-1.1 show the direct conversion of eriodictyol (ERI) to luteolin (LU, left), and naringenin (NAR) to apigenin (AP, right), respectively. (C) Molecular model of the active site of LjFNSII-1.1.
Article link: http://www.nature.com/articles/srep19245
Contact information
Prof. WANG Liangsheng
Key Laboratory of Plant Resources/Beijing Botanical Garden
Institute of Botany, Chinese Academy of Sciences, Beijing 100093, China
E-mail: wanglsh@ibcas.ac.cn
