Dr. Jinxing Lin's Group Reveals Shutoff Control of the Ammonium Transporter AMT1;3 to Avoid Toxic Accumulation
Print |
Close Text size:A A A
Ammonium is a preferred source of nitrogen for plants but is toxic at high levels. Plant ammonium transporters (AMTs) play an essential role in NH4+ uptake, but the mechanism by which AMTs are regulated remains unclear.
To study how AMTs are regulated in the presence of ammonium, Dr. Jinxing Lin’s group used variable-angle total internal reflection fluorescence microscopy and fluorescence cross-correlation spectroscopy for single-particle fluorescence imaging of EGFP-tagged AMT1;3 on the plasma membrane of Arabidopsis root cells at various ammonium levels. It was revealed that AMT1;3-EGFP dynamically appeared and disappeared on the plasma membrane as moving fluorescent spots in low oligomeric states under N-deprived and N-sufficient conditions. Under external high-ammonium stress, however, AMT1;3-EGFPs were found to amass into clusters, which were then internalized into the cytoplasm. A similar phenomenon also occurred in the glutamine synthetase mutant gln1;2 background. Single-particle analysis of AMT1;3-EGFPs in the clathrin heavy chain 2 mutant (chc2 mutant) and Flotllin1 artificial microRNA (Flot1 amiRNA) backgrounds, together with chemical inhibitor treatments, demonstrated that the endocytosis of AMT1;3 clusters induced by high-ammonium stress could occur mainly through clathrin-mediated endocytic pathways, but the contribution of microdomain-associated endocytic pathway cannot be excluded in the internalization. The results demonstrated that the clustering and endocytosis of AMT1;3 provides an effective mechanism by which plant cells can avoid accumulation of toxic levels of ammonium by eliminating active AMT1;3 from the plasma membrane.
This work has been published in PNAS online (Wang et al., 2013, Doi: 10.1073/pnas.1301160110). This work is supported by 973 Program Grants 2011CB944600, 2011CB809103, the Knowledge Innovation Program of the Chinese Academy of Sciences (KSCX2-EW-Q-20-2), and National Nature Science Foundation of China Project Grant (31070326, 30821007).
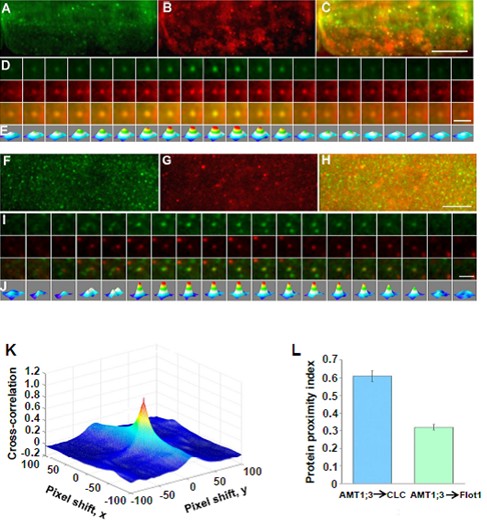
Fig. 6. AMT1;3-EGFP and mCherry-CLC/mCherry-Flot1 spots co-localize at the plasma membrane.
(A)-(E) The co-localize of AMT1;3-EGFP spots with mCherry-CLC particles.
(F)-(J) The co-localize of AMT1;3-EGFP spots with mCherry-Flot1particles.
(K) A typical 3D plot of AMT1;3-EGFP and mCherry-CLC cross-correlation vs. pixel shift.
(L) Mean protein proximity index (PPI) values showing high/low co-localization degree of AMT1;3-EGFP and mCherry-CLC/Flot1.


