Endocytosis is essential for the maintenance of protein and lipid compositions in the plasma membrane and for the acquisition of materials from the extracellular space. Clathrin-dependent and -independent endocytic processes are well established in yeast and animals; however, endocytic pathways involved in cargo internalization and intracellular trafficking remain to be fully elucidated for plants.
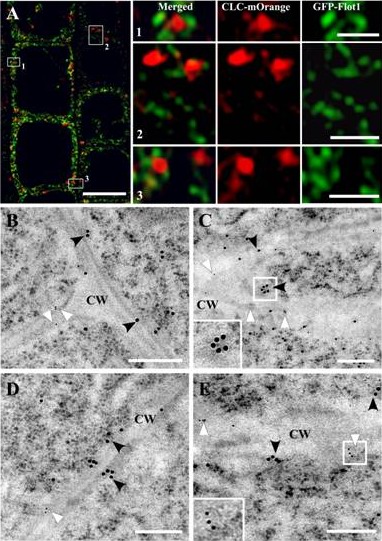
Prof. LIN Jinxing and his research group, Key Laboratory of Plant Molecular Physiology, Institute of Botany, the Chinese Academy of Sciences, used transgenic green fluorescent protein–flotillin1 (GFP-Flot1) Arabidopsis thaliana plants in combination with confocal microscopy analysis and transmission electron microscopy immunogold labeling to study the spatial and dynamic aspects of GFP-Flot1–positive vesicle formation. They found that vesicle size, as outlined by the gold particles, was ~100 nm, which is larger than the 30-nm size of clathrin-coated vesicles. GFP-Flot1 also did not colocalize with clathrin light chain-mOrange. Variable-angle total internal reflection fluorescence microscopy also revealed that the dynamic behavior of GFP-Flot1–positive puncta was different from that of clathrin light chain-mOrange puncta. Furthermore, disruption of membrane microdomains caused a significant alteration in the dynamics of Flot1-positive puncta. Analysis of artificial microRNA Flot1 transgenic Arabidopsis lines established that a reduction in Flot1 transcript levels gave rise to a reduction in shoot and root meristem size plus retardation in seedling growth. Taken together, these findings support the hypothesis that, in plant cells, Flot1 is involved in a clathrin-independent endocytic pathway and functions in seedling development.
The research work was recently published online on the Plant Cell (http://www.plantcell.org/cgi/doi/10.1105/tpc.112.095695). The study was supported by 973 programs, NNSFC projects and CAS programs.
 |
| In Vivo Confocal Fluorescence Analysis and Double Immunogold Electron Microscopy Studies Establish That Flot1 and CLC Are Not Colocalized |
