Although earlier studies focused on the structure and regulation mechanisms of plant aquaporins, few have examined the diffusion dynamics and partitioning of membrane proteins in living cells. The motion of Arabidopsis thaliana PIP2;1 , which is important for bridging data collected at the level of individual molecules to the physical and physiological processes, was studied by Research group lead by professor Jinxing Lin from Institute of Botany, CAS.
Single-particle tracking analysis revealed that PIP2;1 presented four diffusion modes with large dispersion of diffusion coefficients, suggesting that partitioning and dynamics of PIP2;1 are heterogeneous and, more importantly, that PIP2;1 can move into or out of membrane microdomains. In response to salt stress, the diffusion coefficients and percentage of restricted diffusion increased, implying that PIP2;1 internalization was enhanced. This was further supported by the decrease in PIP2;1 density on plasma membranes by FCS. We additionally demonstrated that PIP2;1 internalization involves a combination of two pathways: a tyrphostin A23-sensitive clathrin-dependent pathway and a methyl-β-cyclodextrin–sensitive, membrane raft–associated pathway. The latter was efficiently stimulated under NaCl conditions. Taken together, the researchers’ findings demonstrate that PIP2;1 molecules are heterogeneously distributed on the plasma membrane and that clathrin and membrane raft pathways cooperate to mediate the subcellular trafficking of PIP2;1, suggesting that the dynamic partitioning and recycling pathways might be involved in the multiple modes of regulating water permeability.
The research work was recently published online on the Plant Cell (http://www.plantcell.org/content/early/2011/10/18/tpc.111.091454.full.pdf+html ). The study was supported by 973 programs, NNSFC projects and CAS programs.
Contacts: Dr. Jinxing Lin, linjx@ibcas.ac.cn
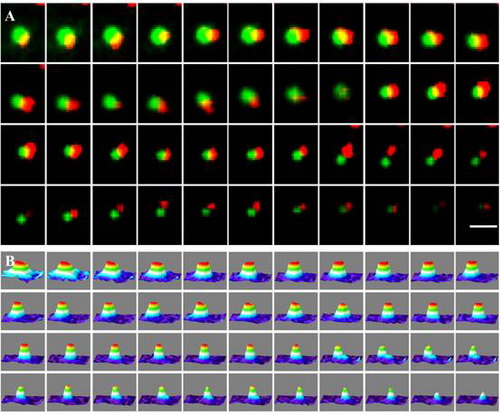
Time lapse showing an example of one overlapped GFP-PIP2;1 and mCherry-CLC spot codiffusion away from the focus, indicating that the internalization of PIP2;1was associated with the clathrin-dependent pathway. A. EWM image of overlapped clusters proximal to the plasma membrane; B. Three-dimensional luminance plots of the corresponding spots in A.
Although earlier studies focused on the structure and regulation mechanisms of plant aquaporins, few have examined the diffusion dynamics and partitioning of membrane proteins in living cells. The motion of Arabidopsis thaliana PIP2;1 , which is important for bridging data collected at the level of individual molecules to the physical and physiological processes, was studied by Research group lead by professor Jinxing Lin from Institute of Botany, CAS.
Single-particle tracking analysis revealed that PIP2;1 presented four diffusion modes with large dispersion of diffusion coefficients, suggesting that partitioning and dynamics of PIP2;1 are heterogeneous and, more importantly, that PIP2;1 can move into or out of membrane microdomains. In response to salt stress, the diffusion coefficients and percentage of restricted diffusion increased, implying that PIP2;1 internalization was enhanced. This was further supported by the decrease in PIP2;1 density on plasma membranes by FCS. We additionally demonstrated that PIP2;1 internalization involves a combination of two pathways: a tyrphostin A23-sensitive clathrin-dependent pathway and a methyl-β-cyclodextrin–sensitive, membrane raft–associated pathway. The latter was efficiently stimulated under NaCl conditions. Taken together, the researchers’ findings demonstrate that PIP2;1 molecules are heterogeneously distributed on the plasma membrane and that clathrin and membrane raft pathways cooperate to mediate the subcellular trafficking of PIP2;1, suggesting that the dynamic partitioning and recycling pathways might be involved in the multiple modes of regulating water permeability.
The research work was recently published online on the Plant Cell (http://www.plantcell.org/content/early/2011/10/18/tpc.111.091454.full.pdf+html ). The study was supported by 973 programs, NNSFC projects and CAS programs.
Contacts: Dr. Jinxing Lin, linjx@ibcas.ac.cn
 |
| Time lapse showing an example of one overlapped GFP-PIP2;1 and mCherry-CLC spot codiffusion away from the focus, indicating that the internalization of PIP2;1was associated with the clathrin-dependent pathway. A. EWM image of overlapped clusters proximal to the plasma membrane; B. Three-dimensional luminance plots of the corresponding spots in A. |
