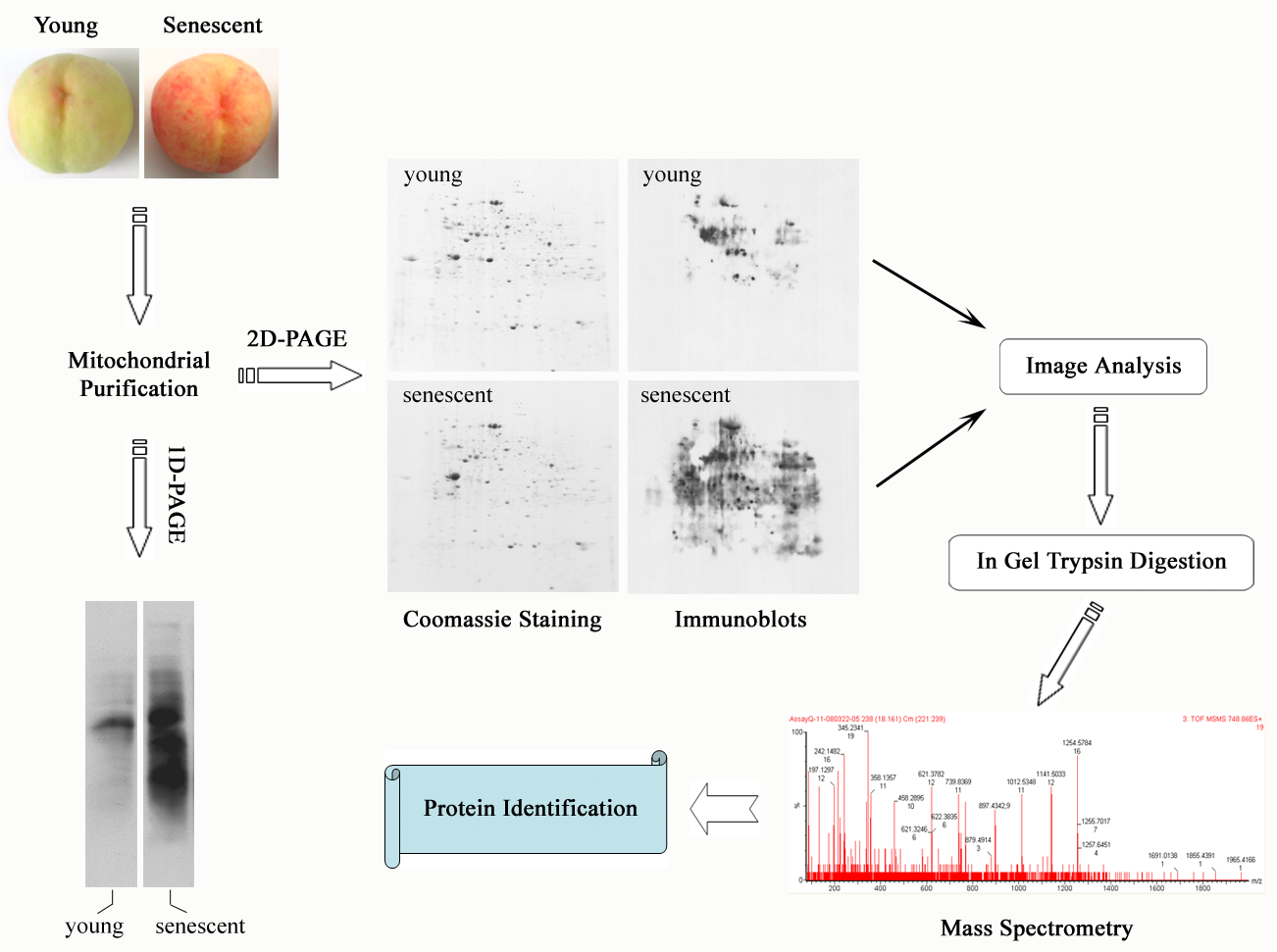
Oxidative Damage of Mitochondrial Proteins Contributes to Fruit Senescence: A Redox Proteomics Analysis
Qin GZ, Meng XH, Wang Q, Tian SP
Journal of Proteome Research
Vol. 8 Issue 5 Page 2449-2462
Impact Factor: 5.675
Abstract:
Oxidative damage to mitochondria caused by reactive oxygen species (ROS) has been implicated in the process of senescence as well as a number of senescence-related disorders in a variety of organisms. Whereas mitochondrial DNA was shown to be oxidatively modified during cellular senescence, mitochondrial protein oxidation is not well-understood. With the use of high-resolution, two-dimensional gel electrophoresis coupled with immunoblotting, we show here that protein carbonylation, a widely used marker of protein oxidation, increased in mitochondria during the senescence of peach fruit. Specific mitochondrial proteins including outer membrane transporter (voltage-dependent anion-selective channel, VDAC), tricarboxylic acid cycle enzymes (malate dehydrogenase and aconitase), and antioxidant proteins (manganese superoxide dismutase, MnSOD) were found as the targets. The oxidative modification was concomitant with a change of VDAC function and loss of catalytic activity of malate dehydrogenase and MnSOD, which in turn facilitated the release of superoxide radicals in mitochondria. Reduction of ROS content by lowering the environmental temperature prevented the accumulation of protein carbonylation in mitochondria and retarded fruit senescence, whereas treatment of fruit with H2O2 had the opposite effect. Our data suggest that oxidative damage of specific mitochondrial proteins may be responsible for impairment of mitochondrial function, thus, leading to fruit senescence. Proteomics analysis of mitochondrial redox proteins provides considerable information on the molecular mechanisms involved in the progression of fruit senescence.
Qin GZ, Meng XH, Wang Q, Tian SP
Journal of Proteome Research
Vol. 8 Issue 5 Page 2449-2462
Impact Factor: 5.675
Abstract:
Oxidative damage to mitochondria caused by reactive oxygen species (ROS) has been implicated in the process of senescence as well as a number of senescence-related disorders in a variety of organisms. Whereas mitochondrial DNA was shown to be oxidatively modified during cellular senescence, mitochondrial protein oxidation is not well-understood. With the use of high-resolution, two-dimensional gel electrophoresis coupled with immunoblotting, we show here that protein carbonylation, a widely used marker of protein oxidation, increased in mitochondria during the senescence of peach fruit. Specific mitochondrial proteins including outer membrane transporter (voltage-dependent anion-selective channel, VDAC), tricarboxylic acid cycle enzymes (malate dehydrogenase and aconitase), and antioxidant proteins (manganese superoxide dismutase, MnSOD) were found as the targets. The oxidative modification was concomitant with a change of VDAC function and loss of catalytic activity of malate dehydrogenase and MnSOD, which in turn facilitated the release of superoxide radicals in mitochondria. Reduction of ROS content by lowering the environmental temperature prevented the accumulation of protein carbonylation in mitochondria and retarded fruit senescence, whereas treatment of fruit with H2O2 had the opposite effect. Our data suggest that oxidative damage of specific mitochondrial proteins may be responsible for impairment of mitochondrial function, thus, leading to fruit senescence. Proteomics analysis of mitochondrial redox proteins provides considerable information on the molecular mechanisms involved in the progression of fruit senescence.