Nitric oxide modulates the influx of extracellular Ca2+ and actin filament organization during cell wall construction in Pinus bungeana pollen tubes
Wang YH, Chen T, Zhang CY, Hao HQ, Liu P, Zheng MZ, Baluska F, Samaj J, Lin JX
New Phytologist 182(4): 851-865
Impact factor :5.249
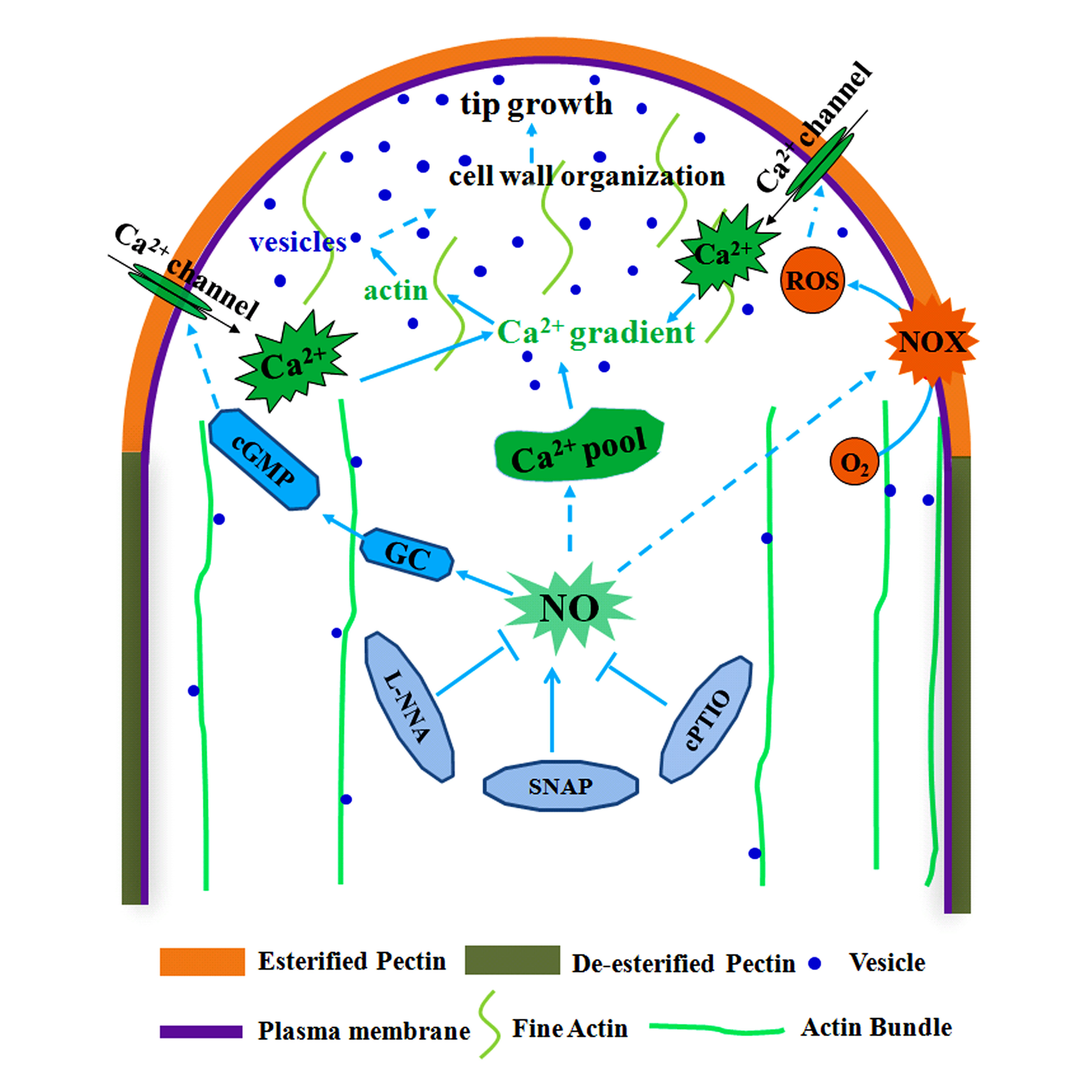
Abstract : Nitric oxide (NO) plays a key role in many physiological processes in plants, including pollen tube growth. Here, effects of NO on extracellular Ca2+ flux and microfilaments during cell wall construction in Pinus bungeana pollen tubes were investigated.Extracellular Ca2+ influx, the intracellular Ca2+ gradient, patterns of actin organization, vesicle trafficking and cell wall deposition upon treatment with the NO donor S-nitroso-N-acetylpenicillamine (SNAP), the NO synthase (NOS) inhibitor N.-nitro-L-arginine (L-NNA) or the NO scavenger 2-(4-carboxyphenyl)-4, 4, 5, 5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO) were analyzed.SNAP enhanced pollen tube growth in a dose-dependent manner, while L-NNA and cPTIO inhibited NO production and arrested pollen tube growth. Noninvasive detection and microinjection of a Ca2+ indicator revealed that SNAP promoted extracellular Ca2+ influx and increased the steepness of the tip-focused Ca2+ gradient, while cPTIO and L-NNA had the opposite effect. Fluorescence labeling indicated that SNAP, cPTIO and L-NNA altered actin organization, which subsequently affected vesicle trafficking. Finally, the configuration and/or distribution of cell wall components such as pectins and callose were significantly altered in response to L-NNA. Fourier transform infrared (FTIR) microspectroscopy confirmed the changes in the chemical composition of walls.Our results indicate that NO affects the configuration and distribution of cell wall components in pollen tubes by altering extracellular Ca2+ influx and F-actin organization.
Wang YH, Chen T, Zhang CY, Hao HQ, Liu P, Zheng MZ, Baluska F, Samaj J, Lin JX
New Phytologist 182(4): 851-865
Impact factor :5.249
Abstract : Nitric oxide (NO) plays a key role in many physiological processes in plants, including pollen tube growth. Here, effects of NO on extracellular Ca2+ flux and microfilaments during cell wall construction in Pinus bungeana pollen tubes were investigated.Extracellular Ca2+ influx, the intracellular Ca2+ gradient, patterns of actin organization, vesicle trafficking and cell wall deposition upon treatment with the NO donor S-nitroso-N-acetylpenicillamine (SNAP), the NO synthase (NOS) inhibitor N.-nitro-L-arginine (L-NNA) or the NO scavenger 2-(4-carboxyphenyl)-4, 4, 5, 5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO) were analyzed.SNAP enhanced pollen tube growth in a dose-dependent manner, while L-NNA and cPTIO inhibited NO production and arrested pollen tube growth. Noninvasive detection and microinjection of a Ca2+ indicator revealed that SNAP promoted extracellular Ca2+ influx and increased the steepness of the tip-focused Ca2+ gradient, while cPTIO and L-NNA had the opposite effect. Fluorescence labeling indicated that SNAP, cPTIO and L-NNA altered actin organization, which subsequently affected vesicle trafficking. Finally, the configuration and/or distribution of cell wall components such as pectins and callose were significantly altered in response to L-NNA. Fourier transform infrared (FTIR) microspectroscopy confirmed the changes in the chemical composition of walls.Our results indicate that NO affects the configuration and distribution of cell wall components in pollen tubes by altering extracellular Ca2+ influx and F-actin organization.

