Elucidating the molecular mechanism of photosystem II (PSII) assembly
Print |
Close Text size:A A A
Prof. Lixin Zhang's group has made progress in elucidating the molecular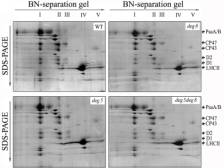 mechanisms of regulation of photosystem II function. Characterization of the lpa mutant of Arabidopsis thaliana , which generally accumulates lower than wild-type levels of the PSII complex, revealed the molecular chaperones in the assembly of PSII. LPA1 appears to be an integral membrane chaperone required for efficient PSII assembly, probably through direct interaction with the PSII reaction center protein D1 (Plant Cell, 2006). LPA2 forms a complex with Alb3, which assists CP43 assembly within PSII (Plant Cell, 2007a). DEG5 and DEG8 were found to form a hexamer in the thylakoid lumen, and recombinant DEG8 could be proteolytically active towards photodamaged D1 protein of PSII, producing 16-kD N-terminal and 18-kD C-terminal fragments. These results suggest that DEG5 and DEG8 have a synergistic function in the primary cleavage of the CD loop of the PSII reaction center protein D1 (Plant Cell, 2007b).
mechanisms of regulation of photosystem II function. Characterization of the lpa mutant of Arabidopsis thaliana , which generally accumulates lower than wild-type levels of the PSII complex, revealed the molecular chaperones in the assembly of PSII. LPA1 appears to be an integral membrane chaperone required for efficient PSII assembly, probably through direct interaction with the PSII reaction center protein D1 (Plant Cell, 2006). LPA2 forms a complex with Alb3, which assists CP43 assembly within PSII (Plant Cell, 2007a). DEG5 and DEG8 were found to form a hexamer in the thylakoid lumen, and recombinant DEG8 could be proteolytically active towards photodamaged D1 protein of PSII, producing 16-kD N-terminal and 18-kD C-terminal fragments. These results suggest that DEG5 and DEG8 have a synergistic function in the primary cleavage of the CD loop of the PSII reaction center protein D1 (Plant Cell, 2007b).

 mechanisms of regulation of photosystem II function. Characterization of the lpa mutant of Arabidopsis thaliana , which generally accumulates lower than wild-type levels of the PSII complex, revealed the molecular chaperones in the assembly of PSII. LPA1 appears to be an integral membrane chaperone required for efficient PSII assembly, probably through direct interaction with the PSII reaction center protein D1 (Plant Cell, 2006). LPA2 forms a complex with Alb3, which assists CP43 assembly within PSII (Plant Cell, 2007a). DEG5 and DEG8 were found to form a hexamer in the thylakoid lumen, and recombinant DEG8 could be proteolytically active towards photodamaged D1 protein of PSII, producing 16-kD N-terminal and 18-kD C-terminal fragments. These results suggest that DEG5 and DEG8 have a synergistic function in the primary cleavage of the CD loop of the PSII reaction center protein D1 (Plant Cell, 2007b).
mechanisms of regulation of photosystem II function. Characterization of the lpa mutant of Arabidopsis thaliana , which generally accumulates lower than wild-type levels of the PSII complex, revealed the molecular chaperones in the assembly of PSII. LPA1 appears to be an integral membrane chaperone required for efficient PSII assembly, probably through direct interaction with the PSII reaction center protein D1 (Plant Cell, 2006). LPA2 forms a complex with Alb3, which assists CP43 assembly within PSII (Plant Cell, 2007a). DEG5 and DEG8 were found to form a hexamer in the thylakoid lumen, and recombinant DEG8 could be proteolytically active towards photodamaged D1 protein of PSII, producing 16-kD N-terminal and 18-kD C-terminal fragments. These results suggest that DEG5 and DEG8 have a synergistic function in the primary cleavage of the CD loop of the PSII reaction center protein D1 (Plant Cell, 2007b).