Photosystem II (PSII) is a multisubunit pigment-protein complex and catalyzes light-driven water oxidation, leading to the conversion of light energy into chemical energy and the release of molecular oxygen, proton and electron.
A number of auxiliary factors have been identified that take part in pathways of PSII assembly and repair. Psb27, a small thylakoid lumen-localized protein is one of the well-studied assembly factors with important role in the efficient assembly/repair of PSII. However, the exact location and binding site of Psb27 in the intermediate PSII remain elusive.
A research team from the key laboratory of Photobiology, Institute of Botany, Chinese Academy of Science, and their collaborator from Tsinghua University solved the structure of a PSII assembly/repair intermediate, the Psb27-PSII complex from a thermophilic cyanobacterium Thermosynechococcus vulcanus by cryo-electron microscopy. The study entitled “Structural insights into a dimeric Psb27-photosystem II complex from a cyanobacterium Thermosynechococcus vulcanus” is published in PNAS on January 26th, 2021.
This study showed that Psb27 is associated with CP43 at the lumenal side of PSII, with specific interactions formed between helixes 2, 3 of Psb27 and a loop region between helix III and helix IV of CP43 as well as the large E-loop of CP43.
The binding of Psb27 imposes some structural conflicts with the N-terminal region of PsbO, and also induces some conformational changes in CP43, CP47 and D2. This leads PsbO unable to bind in the Psb27-PSII. Based on the structural information, a schematic model for the assembly of the Mn4CaO5 cluster and extrinsic proteins in the lumenal side of PSII was proposed.
This study provides important structural insights into the regulation mechanism of Psb27 in the biogenesis and repair of PSII, and is a big step forward to understanding the structure-function dynamics during the assembly and repair of PSII.
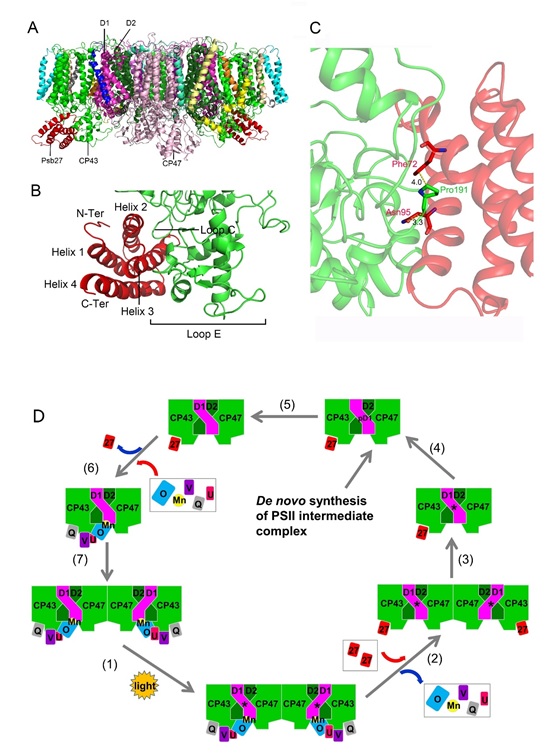
Overall structure of the Psb27-PSII complex from T. vulcanus and a schematic model for the PSII repair/assembly.(Image by IBCAS and Tsinghua University)
Article link: https://www.pnas.org/content/118/5/e2018053118
Contact:Email:hanguangye@ibcas.ac.cn
Institute of Botany, Chinese Academy of Sciences
Photosystem II (PSII) is a multisubunit pigment-protein complex and catalyzes light-driven water oxidation, leading to the conversion of light energy into chemical energy and the release of molecular oxygen, proton and electron.
A number of auxiliary factors have been identified that take part in pathways of PSII assembly and repair. Psb27, a small thylakoid lumen-localized protein is one of the well-studied assembly factors with important role in the efficient assembly/repair of PSII. However, the exact location and binding site of Psb27 in the intermediate PSII remain elusive.
A research team from the key laboratory of Photobiology, Institute of Botany, Chinese Academy of Science, and their collaborator from Tsinghua University solved the structure of a PSII assembly/repair intermediate, the Psb27-PSII complex from a thermophilic cyanobacterium Thermosynechococcus vulcanus by cryo-electron microscopy. The study entitled “Structural insights into a dimeric Psb27-photosystem II complex from a cyanobacterium Thermosynechococcus vulcanus” is published in PNAS on January 26th, 2021.
This study showed that Psb27 is associated with CP43 at the lumenal side of PSII, with specific interactions formed between helixes 2, 3 of Psb27 and a loop region between helix III and helix IV of CP43 as well as the large E-loop of CP43.
The binding of Psb27 imposes some structural conflicts with the N-terminal region of PsbO, and also induces some conformational changes in CP43, CP47 and D2. This leads PsbO unable to bind in the Psb27-PSII. Based on the structural information, a schematic model for the assembly of the Mn4CaO5 cluster and extrinsic proteins in the lumenal side of PSII was proposed.
This study provides important structural insights into the regulation mechanism of Psb27 in the biogenesis and repair of PSII, and is a big step forward to understanding the structure-function dynamics during the assembly and repair of PSII.

Overall structure of the Psb27-PSII complex from T. vulcanus and a schematic model for the PSII repair/assembly.(Image by IBCAS and Tsinghua University)
Article link: https://www.pnas.org/content/118/5/e2018053118
Contact:Email:hanguangye@ibcas.ac.cn
Institute of Botany, Chinese Academy of Sciences
