During host–pathogen coevolution, plants have evolved intracellular-localized NOD-like receptors (NLRs) that monitor pathogen effectors and trigger effector-triggered immunity (ETI).
Overexpression of NLRs (i.e. SUPPRESSOR OF npr1-1, CONSTITUTIVE 1, SNC1) or its interacting proteins (i.e. an SNC1-interacting protein TOPLESS-RELATED 1, TPR1) results in autoimmunity, which reduces plant growth and development.
Therefore, NLR protein-mediated immune responses are tightly regulated to avoid autoimmunity and reduced plant vigor. For example, SKP1-CULLIN1-F-box (SCF)-mediated protein degradation maintains NLR protein levels below the threshold that would trigger autoimmunity under non-pathogenic conditions.
Recent studies suggest that a SUMO (Small Ubiquitin-related Modifier) E3 ligase SIZ1-mediated SUMO modification may represses SNC1-mediated immune response, but the detailed molecular mechanism remains to be elucidated. Covalent conjugation of a SUMO to the lysine residue(s) of a target protein, a process referred to as SUMOylation, regulates protein stability, subcellular localization, enzymatic activity, and/or protein–protein interactions.
Recently, Jingbo Jin’s research group from Institute of Botany, Chinese Academy of Sciences, found that SIZ1-mediated SUMOylation of TPR1 represses plant immunity, which at least partly contribute to the suppression of autoimmunity under non-pathogenic conditions to ensure proper plant development.
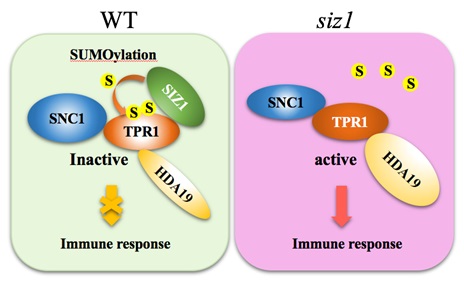
It has been known that TPR1, a transcriptional corepressor, positively regulates plant immunity by interacting with SNC1 and histone deacetylase 19 (HDA19).
The research has found that SIZ1 mediates SUMOylation of TPR1, and the K282 and K721 residues in TPR1 serve as critical SUMO attachment sites.
Simultaneous K282R and K721R substitutions in TPR1 blocked its SUMOylation, enhanced its transcriptional co-repressor activity, increased TPR1-HDA19 interaction, and inhibited TPR1-SNC1 interaction, suggesting that SUMOylation of TPR1 represses its transcriptional co-repressor activity, inhibits its interaction with HDA19, and traps SNC1 in a resting state in a complex with TPR1 under non-pathogenic conditions.
Thus, repression of TPR1 activity by SUMO modification is an important mechanism inhibiting the constitutive activation of SNC1-dependent immune responses under non-pathogenic conditions.
This finding has been published in Molecular Plant entitled "SIZ1-Mediated SUMOylation of TPR1 Suppresses Plant Immunity in Arabidopsis".
This work was supported by the Chinese Academy of Sciences and the National Natural Science Foundation of China.
Article Link:https://doi.org/10.1016/j.molp.2018.12.002
CONTAC INFO:
Institute of Botany, Chinese Academy of Sciences,
20 Nanxincun, Xiangshan, Beijing 100093, China
E-mail: Int_office@ibcas.ac.cn
During host–pathogen coevolution, plants have evolved intracellular-localized NOD-like receptors (NLRs) that monitor pathogen effectors and trigger effector-triggered immunity (ETI).
Overexpression of NLRs (i.e. SUPPRESSOR OF npr1-1, CONSTITUTIVE 1, SNC1) or its interacting proteins (i.e. an SNC1-interacting protein TOPLESS-RELATED 1, TPR1) results in autoimmunity, which reduces plant growth and development.
Therefore, NLR protein-mediated immune responses are tightly regulated to avoid autoimmunity and reduced plant vigor. For example, SKP1-CULLIN1-F-box (SCF)-mediated protein degradation maintains NLR protein levels below the threshold that would trigger autoimmunity under non-pathogenic conditions.
Recent studies suggest that a SUMO (Small Ubiquitin-related Modifier) E3 ligase SIZ1-mediated SUMO modification may represses SNC1-mediated immune response, but the detailed molecular mechanism remains to be elucidated. Covalent conjugation of a SUMO to the lysine residue(s) of a target protein, a process referred to as SUMOylation, regulates protein stability, subcellular localization, enzymatic activity, and/or protein–protein interactions.
Recently, Jingbo Jin’s research group from Institute of Botany, Chinese Academy of Sciences, found that SIZ1-mediated SUMOylation of TPR1 represses plant immunity, which at least partly contribute to the suppression of autoimmunity under non-pathogenic conditions to ensure proper plant development.
It has been known that TPR1, a transcriptional corepressor, positively regulates plant immunity by interacting with SNC1 and histone deacetylase 19 (HDA19).
The research has found that SIZ1 mediates SUMOylation of TPR1, and the K282 and K721 residues in TPR1 serve as critical SUMO attachment sites.
Simultaneous K282R and K721R substitutions in TPR1 blocked its SUMOylation, enhanced its transcriptional co-repressor activity, increased TPR1-HDA19 interaction, and inhibited TPR1-SNC1 interaction, suggesting that SUMOylation of TPR1 represses its transcriptional co-repressor activity, inhibits its interaction with HDA19, and traps SNC1 in a resting state in a complex with TPR1 under non-pathogenic conditions.
Thus, repression of TPR1 activity by SUMO modification is an important mechanism inhibiting the constitutive activation of SNC1-dependent immune responses under non-pathogenic conditions.
This finding has been published in Molecular Plant entitled "SIZ1-Mediated SUMOylation of TPR1 Suppresses Plant Immunity in Arabidopsis".
This work was supported by the Chinese Academy of Sciences and the National Natural Science Foundation of China.
Article Link:https://doi.org/10.1016/j.molp.2018.12.002
CONTAC INFO:
Institute of Botany, Chinese Academy of Sciences,
20 Nanxincun, Xiangshan, Beijing 100093, China
E-mail: Int_office@ibcas.ac.cn