Red algae are one of the most primitive eukaryotic algae closely related to prokaryotic cyanobacteria, and have a unique evolutionary position between prokaryotic and eukaryotic organisms.
Photosystem I (PSI) is one of the two photosystems present in oxygenic photosynthetic organisms, and function to harvest and convert light energy into chemical energy in photosynthesis. PSI is known as one of the most efficient nano-photochemical machines in nature.
The crystal structure of cyanobacterial PSI core and higher plant PSI-LHCI has been determined at higher resolution; however, no structure of PSI-LHCI from various algae has been reported so far.
A research group led by Prof. Tingyun Kuang from the Institute of Botany, Chinese Academy of Sciences, has been studying the structure and function of photosynthetic membrane protein supercomplexes for a long time.
Recently, this group in collaboration with Prof. Sen-Fang Sui of Tsinghua university revealed the structure of PSI-LHCR from a red alga Cyanidioschyzon merolae by single-particle cryo-electron microscopy (cryo-EM) at a resolution of 3.63 ?.
It has two forms of the PSI-LHCR supercomplex, one with 3 Lhcr antenna subunits attached (designated PSI-3Lhcr) and the other one with 5 Lhcrs subunits attached (designated PSI-5Lhcr).
The 3 Lhcr subunits are associated with one side of the PSI core similar to that of the higher plant PSI, whereas the other 2 additional Lhcr subunits are attached to the opposite side in PSI-5Lhcr, indicating an ancient form of PSI-LHCI.
Furthermore, the red algal PSI core showed features of both cyanobacterial and higher plant PSI, suggesting an intermediate type during evolution from prokaryotes to eukaryotes.
Among them, the structure of eukaryotic PsaO was identified for the first time in the PSI core, which binds 3 chlorophylls a and may be important to mediate energy transfer from LHCI to the PSI core.
The study identified individual interaction sites between LHCRs and the core subunits, as well as pigments unique to the LHCRs which are different from those of LHCs in the plant PSI-LHCI.
This study revealed unique energy transfer pathways different from those of higher plant PSI-LHCI, its adaptation to the changing environment, and the possible changes of PSI-LHCI during evolution from prokaryotes to eukaryotes. These results provide important clues to clarifying the evolution and function of PSI from prokaryotic to eukaryotic photosynthetic organisms.
This is another important achievement on the structures and functions of PSI-LHCI since publication of the structure of higher plant PSI-LHCI (Qin et al., Science, 2015) by Profs. Tingyun Kuang and their colleagues.
The results were published in a paper entitled “Unique organization of photosystem I-light harvesting supercomplex revealed by cryo-EM from a red alga” online recently in the journal Proceedings of the National Academy of Sciences of the United States of America.
This work was supported by funds from the National Key R&D Program of China, the National Basic Research Program of China, the National Natural Science Foundation of China, a Strategic Priority Research Program of CAS and a CAS Key Research Project for Frontier Science.
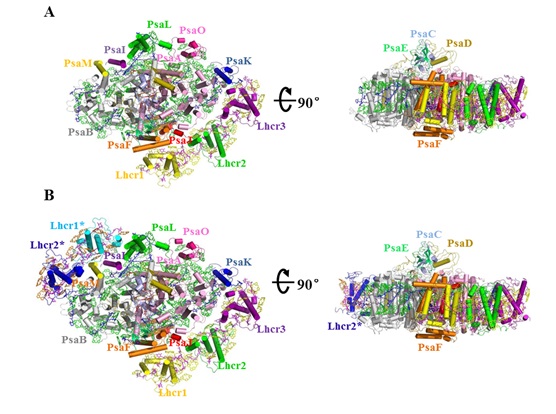
Two forms of the PSI-LHCR supercomplex from C. merolae. (A) Structure of PSI-3Lhcr viewed along the membrane normal from the stromal side and its side view. (B) Structure of PSI-5Lhcr viewed along the membrane normal from the stromal side and its side view.
CONTAC INFO:
Email:Int_office@ibcas.ac.cn
Key Laboratory of Photobiology, CAS
Institute of Botany, Chinese Academy of Sciences,
20 Nanxincun, Xiangshan, Beijing 100093, China
Red algae are one of the most primitive eukaryotic algae closely related to prokaryotic cyanobacteria, and have a unique evolutionary position between prokaryotic and eukaryotic organisms.
Photosystem I (PSI) is one of the two photosystems present in oxygenic photosynthetic organisms, and function to harvest and convert light energy into chemical energy in photosynthesis. PSI is known as one of the most efficient nano-photochemical machines in nature.
The crystal structure of cyanobacterial PSI core and higher plant PSI-LHCI has been determined at higher resolution; however, no structure of PSI-LHCI from various algae has been reported so far.
A research group led by Prof. Tingyun Kuang from the Institute of Botany, Chinese Academy of Sciences, has been studying the structure and function of photosynthetic membrane protein supercomplexes for a long time.
Recently, this group in collaboration with Prof. Sen-Fang Sui of Tsinghua university revealed the structure of PSI-LHCR from a red alga Cyanidioschyzon merolae by single-particle cryo-electron microscopy (cryo-EM) at a resolution of 3.63 ?.
It has two forms of the PSI-LHCR supercomplex, one with 3 Lhcr antenna subunits attached (designated PSI-3Lhcr) and the other one with 5 Lhcrs subunits attached (designated PSI-5Lhcr).
The 3 Lhcr subunits are associated with one side of the PSI core similar to that of the higher plant PSI, whereas the other 2 additional Lhcr subunits are attached to the opposite side in PSI-5Lhcr, indicating an ancient form of PSI-LHCI.
Furthermore, the red algal PSI core showed features of both cyanobacterial and higher plant PSI, suggesting an intermediate type during evolution from prokaryotes to eukaryotes.
Among them, the structure of eukaryotic PsaO was identified for the first time in the PSI core, which binds 3 chlorophylls a and may be important to mediate energy transfer from LHCI to the PSI core.
The study identified individual interaction sites between LHCRs and the core subunits, as well as pigments unique to the LHCRs which are different from those of LHCs in the plant PSI-LHCI.
This study revealed unique energy transfer pathways different from those of higher plant PSI-LHCI, its adaptation to the changing environment, and the possible changes of PSI-LHCI during evolution from prokaryotes to eukaryotes. These results provide important clues to clarifying the evolution and function of PSI from prokaryotic to eukaryotic photosynthetic organisms.
This is another important achievement on the structures and functions of PSI-LHCI since publication of the structure of higher plant PSI-LHCI (Qin et al., Science, 2015) by Profs. Tingyun Kuang and their colleagues.
The results were published in a paper entitled “Unique organization of photosystem I-light harvesting supercomplex revealed by cryo-EM from a red alga” online recently in the journal Proceedings of the National Academy of Sciences of the United States of America.
This work was supported by funds from the National Key R&D Program of China, the National Basic Research Program of China, the National Natural Science Foundation of China, a Strategic Priority Research Program of CAS and a CAS Key Research Project for Frontier Science.

Two forms of the PSI-LHCR supercomplex from C. merolae. (A) Structure of PSI-3Lhcr viewed along the membrane normal from the stromal side and its side view. (B) Structure of PSI-5Lhcr viewed along the membrane normal from the stromal side and its side view.
CONTAC INFO:
Email:Int_office@ibcas.ac.cn
Key Laboratory of Photobiology, CAS
Institute of Botany, Chinese Academy of Sciences,
20 Nanxincun, Xiangshan, Beijing 100093, China
