Plants synthesize more than 200, 000 structurally diversified metabolites. There are over 20, 000 terpenoids with known structures. Many terpenoids play important roles in plant growth and development, and adaptation to the environments.
Triterpenoids, such as triterpenoid saponins from Panax ginseng and Panax notoginseng, are the major bioactive compositions for these medicine plants. Plant 2, 3-oxidosqualene cyclases (OSC) is the key metabolic diverse enzyme that catalyze 2,3-oxidosqualene into more than 100 triterpene skeletons with different conformation and divergent structures.
However the intensive study on OSC enzyme family, the mechanism of rising diverse triterpenes is not well understood.
Research group led by Prof. QI Xiaoquan from Key Laboratory of Plant Molecular Physiology, Institute of Botany, Chinese Academy of Sciences (CAS), China, has previously reported that parkeol synthase (OsPS), an OSC from japonica subspecies of cultivated rice (Oryza sativa L.), synthsizes a tetracyclic parkeol with Chair-Boat-Chair (C-B-C) conformation.
Recently the ortholog gene encoding a novel promiscuous OSC, orysatinol synthase (OsOS), was characterized from indica subspecies of rice, and revealed that OsOS synthesizes a novel Chair-Semi-Chair-Chair (C-sC-C) conformational pentacyclic triterpene orysatinol as its main product and over 12 different other triterpenes.
The OsOS genes are widely distributed in indica subspecies of cultivated rice and in wild rice accessions with AA genome, while OsPS genes only sustained in japonica subspecies and its related wild species.
Phylogenetic, molecular evolution, protein structure and biochemical analyses identified three key amino acid residues amongst 46 polymorphic sites that determine functional conversion between OsPS and OsOS, specifically, the C-sC-C and C-B-C conformation interchanges. The different orientation of a fourth amino acid residue Tyr257 was shown to be important for functional conversion.
This study discovered several new triterpenes including orysatinol, and also revealed the mechanistic insights into the cyclization of 2,3-oxidosqualene into tetra- and pentacyclic skeletons with C-B-C and C-sC-C conformations. This provides useful information for design of a specific triterpene synthase when synthetic biology strategy is applied to produce commercial active triterpeniods.
This study entitled “Identification of key amino acid residues determining product specificity of 2,3-oxidosqualene cyclase in Oryza species” has been published in New Phytologist. Dr. XUE Zheyong and Dr. TAN Zhengwei from Institute of Botany, CAS, are the first co-authors, and Prof. QI Xiaoquan from Institute of Botany, CAS, China, and Prof. Anne Osbourn from John Innes Centre, UK, are the corresponding authors.
This work was supported by funds from the National Natural Science Foundation of China, China Scholarship Council, China, and Biotechnology and Biological Sciences Research Council in UK (BBSRC).
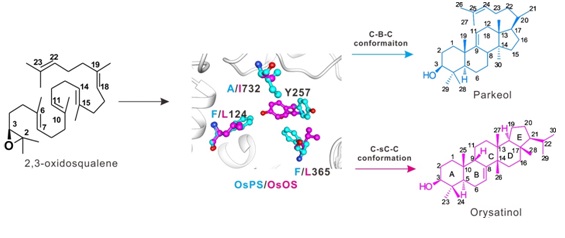
Three key amino acid residues of 2,3-oxidosqualene cyclase (OSC) determine product conformation and specificity through changing orientation of a fourth residue Tyr 257. C-sC-C: Chair-semi-Chair-Chair; C-B-C: Chair-Boat-Chair.
Aticle link: http://onlinelibrary.wiley.com/doi/10.1111/nph.15080/full
CONTAC INFO:
Prof. QI Xiaoquan
Key Laboratory of Plant Molecular Physiology, CAS
Institute of Botany, Chinese Academy of Sciences,
20 Nanxincun, Xiangshan, Beijing 100093, China
E-mail: xqi@ibcas.ac.cn
Plants synthesize more than 200, 000 structurally diversified metabolites. There are over 20, 000 terpenoids with known structures. Many terpenoids play important roles in plant growth and development, and adaptation to the environments.
Triterpenoids, such as triterpenoid saponins from Panax ginseng and Panax notoginseng, are the major bioactive compositions for these medicine plants. Plant 2, 3-oxidosqualene cyclases (OSC) is the key metabolic diverse enzyme that catalyze 2,3-oxidosqualene into more than 100 triterpene skeletons with different conformation and divergent structures.
However the intensive study on OSC enzyme family, the mechanism of rising diverse triterpenes is not well understood.
Research group led by Prof. QI Xiaoquan from Key Laboratory of Plant Molecular Physiology, Institute of Botany, Chinese Academy of Sciences (CAS), China, has previously reported that parkeol synthase (OsPS), an OSC from japonica subspecies of cultivated rice (Oryza sativa L.), synthsizes a tetracyclic parkeol with Chair-Boat-Chair (C-B-C) conformation.
Recently the ortholog gene encoding a novel promiscuous OSC, orysatinol synthase (OsOS), was characterized from indica subspecies of rice, and revealed that OsOS synthesizes a novel Chair-Semi-Chair-Chair (C-sC-C) conformational pentacyclic triterpene orysatinol as its main product and over 12 different other triterpenes.
The OsOS genes are widely distributed in indica subspecies of cultivated rice and in wild rice accessions with AA genome, while OsPS genes only sustained in japonica subspecies and its related wild species.
Phylogenetic, molecular evolution, protein structure and biochemical analyses identified three key amino acid residues amongst 46 polymorphic sites that determine functional conversion between OsPS and OsOS, specifically, the C-sC-C and C-B-C conformation interchanges. The different orientation of a fourth amino acid residue Tyr257 was shown to be important for functional conversion.
This study discovered several new triterpenes including orysatinol, and also revealed the mechanistic insights into the cyclization of 2,3-oxidosqualene into tetra- and pentacyclic skeletons with C-B-C and C-sC-C conformations. This provides useful information for design of a specific triterpene synthase when synthetic biology strategy is applied to produce commercial active triterpeniods.
This study entitled “Identification of key amino acid residues determining product specificity of 2,3-oxidosqualene cyclase in Oryza species” has been published in New Phytologist. Dr. XUE Zheyong and Dr. TAN Zhengwei from Institute of Botany, CAS, are the first co-authors, and Prof. QI Xiaoquan from Institute of Botany, CAS, China, and Prof. Anne Osbourn from John Innes Centre, UK, are the corresponding authors.
This work was supported by funds from the National Natural Science Foundation of China, China Scholarship Council, China, and Biotechnology and Biological Sciences Research Council in UK (BBSRC).

Three key amino acid residues of 2,3-oxidosqualene cyclase (OSC) determine product conformation and specificity through changing orientation of a fourth residue Tyr 257. C-sC-C: Chair-semi-Chair-Chair; C-B-C: Chair-Boat-Chair.
Aticle link: http://onlinelibrary.wiley.com/doi/10.1111/nph.15080/full
CONTAC INFO:
Prof. QI Xiaoquan
Key Laboratory of Plant Molecular Physiology, CAS
Institute of Botany, Chinese Academy of Sciences,
20 Nanxincun, Xiangshan, Beijing 100093, China
E-mail: xqi@ibcas.ac.cn
