Heme and chlorophyll are tetrapyrroles essential for respiration and photosynthesis. Plants synthesize these tetrapyrrole molecules from glutamate, which is attached to tRNA. A V-shaped enzyme, glutamyl-tRNA reductase (GluTR), reduces this glutamate to glutamate-1- semialdehyde (GSA). As a chloroplast stromal enzyme catalyzing the rate-limiting step for tetrapyrrole biosynthesis, GluTR is the target of multiple regulatory factors. The thylakoid membrane protein FLU is a GluTR repressor, and the stromal protein GBP acts as a stimulator. How do these two proteins exert their role on GluTR? Prof. LIU Lin’s team has found that FLU and GBP bind to two spatially apart regions on GluTR (J Biol Chem, 2015, 290: 17559-17565; PNAS, 2014, 111: 6630-6635). Recently, they reported the ternary complex structure of FLU-GluTR-GBP, showing that FLU and GBP can simultaneously bind to GluTR. This work demonstrated that FLU is the anchoring protein for both GluTR and GBP. No direct interaction was detected between GluTR and GSA-aminomutase, the downstream enzyme of GluTR, revealing that GluTR regulation in chloroplasts differs from that in bacteria or archaea.
These results were published on Scientific Reports, 2016, 6: 19756. PhD student FANG Ying is the first author. This work is supported by the National Natural Science Foundation of China, the Ministry of Science and Technology of China, and CAS.
Contact: Prof. LIU Lin
Email: liulin@ibcas.ac.cn
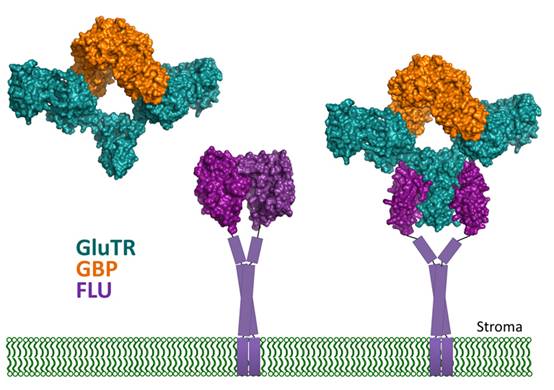
Model of GluTR regulation by GBP and FLU in chloroplasts. The proteins are color-coded, and the lipid bilayer represents the thylakoid membrane.
Heme and chlorophyll are tetrapyrroles essential for respiration and photosynthesis. Plants synthesize these tetrapyrrole molecules from glutamate, which is attached to tRNA. A V-shaped enzyme, glutamyl-tRNA reductase (GluTR), reduces this glutamate to glutamate-1- semialdehyde (GSA). As a chloroplast stromal enzyme catalyzing the rate-limiting step for tetrapyrrole biosynthesis, GluTR is the target of multiple regulatory factors. The thylakoid membrane protein FLU is a GluTR repressor, and the stromal protein GBP acts as a stimulator. How do these two proteins exert their role on GluTR? Prof. LIU Lin’s team has found that FLU and GBP bind to two spatially apart regions on GluTR (J Biol Chem, 2015, 290: 17559-17565; PNAS, 2014, 111: 6630-6635). Recently, they reported the ternary complex structure of FLU-GluTR-GBP, showing that FLU and GBP can simultaneously bind to GluTR. This work demonstrated that FLU is the anchoring protein for both GluTR and GBP. No direct interaction was detected between GluTR and GSA-aminomutase, the downstream enzyme of GluTR, revealing that GluTR regulation in chloroplasts differs from that in bacteria or archaea.
These results were published on Scientific Reports, 2016, 6: 19756. PhD student FANG Ying is the first author. This work is supported by the National Natural Science Foundation of China, the Ministry of Science and Technology of China, and CAS.
Contact: Prof. LIU Lin
Email: liulin@ibcas.ac.cn

