On May 29, 2015, a research team from Institute of Botany, Chinese Academy of Sciences, published an article describing the crystal structure of higher plant PSI-LHCI super-complex at 2.8 ? resolution in the journal Science. This article, entitled "Structural basis for energy transfer pathways in the plant PSI-LHCI super-complex" (Science, 348, 989-995, DOI: 10.1126/science.aab0214), revealed the first atomic view of PSI-LHCI and was highlighted on the cover of the same issue of Science.
Photosynthesis is the largest energy conversion process on earth, and utilizes light energy from the Sun to convert CO2 and water into carbohydrates and oxygen, thus sustaining all aerobic life forms on the earth. Photosynthesis has been considered to be "the most important chemical reaction on Earth". The central problem of photosynthesis is the molecular mechanisms underlying light-harvesting, energy transfer and conversion with high efficiencies.
The energy conversion in photosynthesis is carried out by two large pigment-protein complexes: photosystem I (PSI) and photosystem II (PSII), among which, PSI is an extremely efficient solar energy converter. The light energy absorbed by the PSI light-harvesting pigments is transferred to chlorophylls in the reaction center, where charge separation occurs, and this process produces one electron for nearly every photon absorbed. The high chlorophyll concentration in plant PSI maximizes light harvesting. Furthermore, light-harvesting complex I (LHCI) contain several chlorophylls with red shifted spectra, called red forms, which expand the light-harvesting capacity of plant PSI to the far-red region and allow energy transfer from high-energy to low-energy pigments. The excitation energy transfer in PSI is extremely fast, and the quantum efficiency is close to 100%, making PSI the most efficient energy transfer system. Therefore, the structure and function of PSI has received extensive interests in the field of photosynthesis research. However, the crystal structure of plant PSI-LHCI was solved to a medium resolution so far, therefore the structural basis for the high efficiency of light-harvesting, energy transfer and conversion within plant PSI remained to be elucidated.
The research team has been working on the structure and function of photosynthetic membrane proteins for a long period, and they now resolved the crystal structure of plant PSI-LHCI super-complex at 2.8 ? resolution after many years of efforts. This study revealed the detailed structure of plant PSI-LHCI with a total molecular mass of 600 kDa, which includes 16 subunits (12 core subunits and 4 Lhcas), 155 Chls (143 Chls a and 12 Chls b), 35 carotenoids [26 β-carotenes (BCRs), 5 luteins (Luts) and 4 violaxanthins (Vios)], 10 lipids [6 phosphatidylglycerols (PGs), 3 monogalactosyldiacylglycerols (MGDGs) and 1 digalactosyldiacylglycerol (DGDG)], 3 Fe4S4 clusters, 2 phylloquinones and several water molecules.
Based on the structure resolved, the chlorophyll a and b were differentiated in the 4 Lhca subunits for the first time, and the location and geometrical arrangement of each pigment were revolved, yielding a new pigment network of LHCI. Furthermore, the structure and organization of special chlorophylls - red Chls, in LHCI, were revealed, and 4 plausible energy transfer pathways from LHCI to the PSI core complex were deduced. In addition, detailed differences among the 4 Lhca subunits and their interactions, and the interactions between Lhca subunits and PSI core subunits, were elucidated.
This study provides a solid structural basis for our understanding on the highly efficient energy transfer within the PSI-LHCI super-complex, and thus will be a big step forward toward understanding the mechanisms of photosynthesis. The principles revealed from the natural photosynthesis may prove useful for developing highly efficient solar energy utilization systems,which is important for fulfilling the increasing demand for energy as well as for solving the problems of foods and environment.
Prof. Roberta Croce, an expert in the photosystem I research field, wrote a commentary for this work in the same issue of Science. She commented on this work highly positively in her commentary entitled “A close view of photosystem I” (Science, 348, 970-971). Some public media including Xinhuanet, the Washington Post reported this research work immediately following the publication.
This work was supported by National Basic Research Program of China (Nos. 2011CBA00901, 2015CB150101), a Key Research Program of the Chinese Academy of Sciences (No. KGZD-EW-T05).
Link to the article:
Science, Vol. 348, 989-995,DOI: 10.1126/science.aab0214
Link to the commentary by Prof. Roberta Croce:
Science, Vol. 348, 970-971, DOI: 10.1126/science.aab3387
Link to Xinhuanet:
http://news.xinhuanet.com/tech/2015-05/29/c_1115449197.htm
Link to the Washington Post:
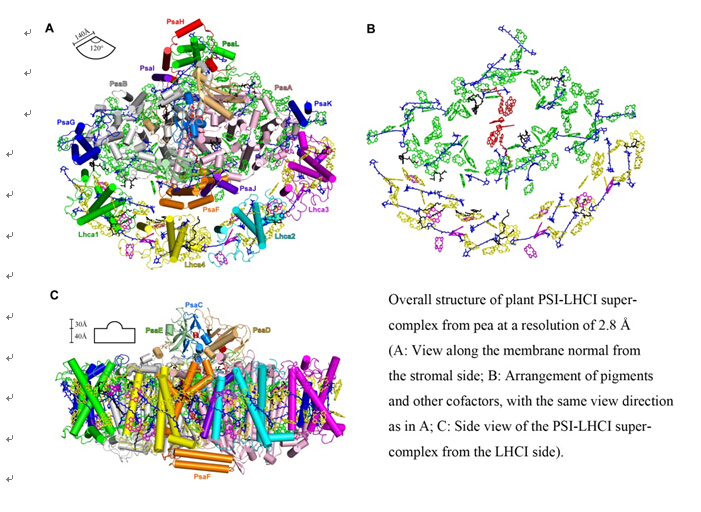

On May 29, 2015, a research team from Institute of Botany, Chinese Academy of Sciences, published an article describing the crystal structure of higher plant PSI-LHCI super-complex at 2.8 ? resolution in the journal Science. This article, entitled "Structural basis for energy transfer pathways in the plant PSI-LHCI super-complex" (Science, 348, 989-995, DOI: 10.1126/science.aab0214), revealed the first atomic view of PSI-LHCI and was highlighted on the cover of the same issue of Science.
Photosynthesis is the largest energy conversion process on earth, and utilizes light energy from the Sun to convert CO2 and water into carbohydrates and oxygen, thus sustaining all aerobic life forms on the earth. Photosynthesis has been considered to be "the most important chemical reaction on Earth". The central problem of photosynthesis is the molecular mechanisms underlying light-harvesting, energy transfer and conversion with high efficiencies.
The energy conversion in photosynthesis is carried out by two large pigment-protein complexes: photosystem I (PSI) and photosystem II (PSII), among which, PSI is an extremely efficient solar energy converter. The light energy absorbed by the PSI light-harvesting pigments is transferred to chlorophylls in the reaction center, where charge separation occurs, and this process produces one electron for nearly every photon absorbed. The high chlorophyll concentration in plant PSI maximizes light harvesting. Furthermore, light-harvesting complex I (LHCI) contain several chlorophylls with red shifted spectra, called red forms, which expand the light-harvesting capacity of plant PSI to the far-red region and allow energy transfer from high-energy to low-energy pigments. The excitation energy transfer in PSI is extremely fast, and the quantum efficiency is close to 100%, making PSI the most efficient energy transfer system. Therefore, the structure and function of PSI has received extensive interests in the field of photosynthesis research. However, the crystal structure of plant PSI-LHCI was solved to a medium resolution so far, therefore the structural basis for the high efficiency of light-harvesting, energy transfer and conversion within plant PSI remained to be elucidated.
The research team has been working on the structure and function of photosynthetic membrane proteins for a long period, and they now resolved the crystal structure of plant PSI-LHCI super-complex at 2.8 ? resolution after many years of efforts. This study revealed the detailed structure of plant PSI-LHCI with a total molecular mass of 600 kDa, which includes 16 subunits (12 core subunits and 4 Lhcas), 155 Chls (143 Chls a and 12 Chls b), 35 carotenoids [26 β-carotenes (BCRs), 5 luteins (Luts) and 4 violaxanthins (Vios)], 10 lipids [6 phosphatidylglycerols (PGs), 3 monogalactosyldiacylglycerols (MGDGs) and 1 digalactosyldiacylglycerol (DGDG)], 3 Fe4S4 clusters, 2 phylloquinones and several water molecules.
Based on the structure resolved, the chlorophyll a and b were differentiated in the 4 Lhca subunits for the first time, and the location and geometrical arrangement of each pigment were revolved, yielding a new pigment network of LHCI. Furthermore, the structure and organization of special chlorophylls - red Chls, in LHCI, were revealed, and 4 plausible energy transfer pathways from LHCI to the PSI core complex were deduced. In addition, detailed differences among the 4 Lhca subunits and their interactions, and the interactions between Lhca subunits and PSI core subunits, were elucidated.
This study provides a solid structural basis for our understanding on the highly efficient energy transfer within the PSI-LHCI super-complex, and thus will be a big step forward toward understanding the mechanisms of photosynthesis. The principles revealed from the natural photosynthesis may prove useful for developing highly efficient solar energy utilization systems,which is important for fulfilling the increasing demand for energy as well as for solving the problems of foods and environment.
Prof. Roberta Croce, an expert in the photosystem I research field, wrote a commentary for this work in the same issue of Science. She commented on this work highly positively in her commentary entitled “A close view of photosystem I” (Science, 348, 970-971). Some public media including Xinhuanet, the Washington Post reported this research work immediately following the publication.
This work was supported by National Basic Research Program of China (Nos. 2011CBA00901, 2015CB150101), a Key Research Program of the Chinese Academy of Sciences (No. KGZD-EW-T05).
Link to the article:
Science, Vol. 348, 989-995,DOI: 10.1126/science.aab0214
Link to the commentary by Prof. Roberta Croce:
Science, Vol. 348, 970-971, DOI: 10.1126/science.aab3387
Link to Xinhuanet:
http://news.xinhuanet.com/tech/2015-05/29/c_1115449197.htm
Link to the Washington Post:


