Sugars not only provide energy for plant growth and development, but also act as signaling molecules to regulate expression of some photosynthesis-related genes. Among the enzymes responsible for sugar metabolism and signaling, Arabidopsis thaliana hexokinase 1 (AtHXK1) plays an essential role in both glycolysis and sugar sensing. Thus, understanding the structural and functional features of AtHXK1 may contribute substantively to the unraveling of sugar metabolic and signaling pathways in plants.
Recently, researchers from the Photosynthesis Center, IBCAS, solved the crystal structures of AtHXK1 in three forms. The glucose-bound and ligand-free forms of AtHXK1 show an "induced-fit" mechanism, and the S177A mutant in glucose-bound form represents a signaling state. Further biochemistry experiments revealed that AtHXK1 follows a sequential substrate binding mechanism. This study presented the first structure of plant hexokinases and the working model for plant HXK1 protein using structural biology methods.
This work entitled "Biochemical and structural study of Arabidopsis hexokinase 1" was published on Acta Crystallographica Section D (doi:10.1107/S1399004714026091) on Feb. 4, 2015. PhD student Feng Juan is the first author, and Profs. Liu Lin and Kuang Tingyun are the co-corresponding authors. This research was funded by 973 Program (MOST), the National Natural Science Foundation of China, and CAS.
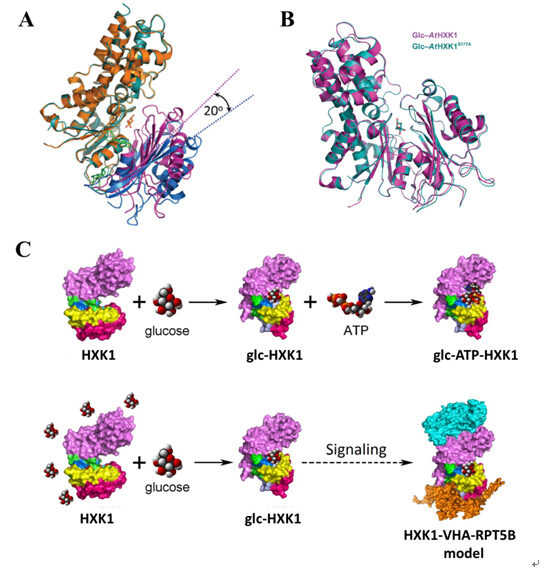
Figure. Crystal structures of AtHXK1 and a model of its dual functions. (A) AtHXK1 in glucose-bound and ligand-free forms (B) Glucose-bound forms of the wild type and the S177A mutant of AtHXK1 (C) A working model for the dual functions of AtHXK1 in glycolysis and sugar signaling.
Sugars not only provide energy for plant growth and development, but also act as signaling molecules to regulate expression of some photosynthesis-related genes. Among the enzymes responsible for sugar metabolism and signaling, Arabidopsis thaliana hexokinase 1 (AtHXK1) plays an essential role in both glycolysis and sugar sensing. Thus, understanding the structural and functional features of AtHXK1 may contribute substantively to the unraveling of sugar metabolic and signaling pathways in plants.
Recently, researchers from the Photosynthesis Center, IBCAS, solved the crystal structures of AtHXK1 in three forms. The glucose-bound and ligand-free forms of AtHXK1 show an "induced-fit" mechanism, and the S177A mutant in glucose-bound form represents a signaling state. Further biochemistry experiments revealed that AtHXK1 follows a sequential substrate binding mechanism. This study presented the first structure of plant hexokinases and the working model for plant HXK1 protein using structural biology methods.
This work entitled "Biochemical and structural study of Arabidopsis hexokinase 1" was published on Acta Crystallographica Section D (doi:10.1107/S1399004714026091) on Feb. 4, 2015. PhD student Feng Juan is the first author, and Profs. Liu Lin and Kuang Tingyun are the co-corresponding authors. This research was funded by 973 Program (MOST), the National Natural Science Foundation of China, and CAS.

Figure. Crystal structures of AtHXK1 and a model of its dual functions. (A) AtHXK1 in glucose-bound and ligand-free forms (B) Glucose-bound forms of the wild type and the S177A mutant of AtHXK1 (C) A working model for the dual functions of AtHXK1 in glycolysis and sugar signaling.
