Chlorophyll is the major pigment that plays an important role in absorbing and transferring light energy during photosynthesis. The biosynthesis of chlorophyll is accomplished by a series of catalysis. The glutamyl-tRNA reductase (GluTR)-catalyzed reduction of glutamyl-tRNA is the rate-limiting and a pivotal regulation step in the chlorophyll biosynthetic pathway. In chloroplast-containing photosynthetic organisms, GluTR-binding protein (GluBP) is a recently identified spatial regulator that allocates GluTR for synthesis of different tetrapyrrole products. However, the underlying molecular mechanisms remain largely unclear.
Dr. Liu Lin and her colleagues from the Institute of Botany, Chinese Academy of Sciences (CAS), and the Institute of Biophysics, CAS, find that GluBP stimulates GluTR catalytic efficiency. The crystal structure of the GluTR-GluBP complex shows that GluBP binding promotes GluTR to a hydride-transferring state, revealing structural details for the catalytic process. These findings clarify a series of arguments regarding the activation and regulation of GluTR. The GluBP structure also suggests that GluBP may have a novel role in heme metabolism.
These results has been online published on Proceeding of National Academy of Sciences, April 21, 2014, and titled: “Crystal Structure of Arabidopsis Glutamyl-tRNA Reductase in Complex with Its Stimulator Protein”. PhD candidates Zhao Aiguo and Fang Ying are the co-first authors, and Dr. Liu Lin is the corresponding author.
This project was supported by National Natural Science Foundation of China, Ministry of Science and Technology, and CAS.
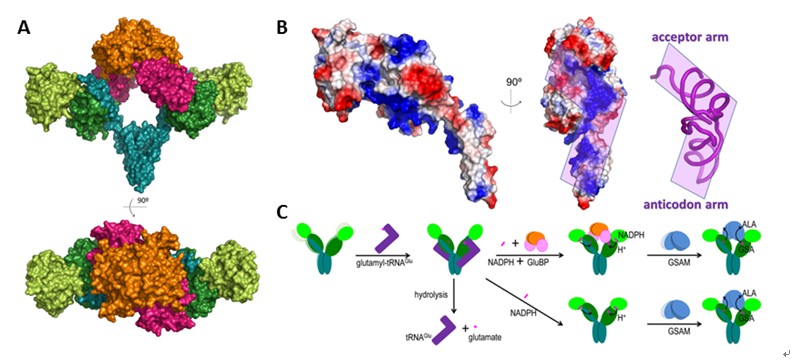
Structure of the GluTR-GluBP complex and a GluTR catalysis model. (A) The side view and top view of the GluTR-GluBP complex structure; (B) Surface potential of GluTR and the predicted tRNA-binding site; (C) Model of GluTR catalysis.
Chlorophyll is the major pigment that plays an important role in absorbing and transferring light energy during photosynthesis. The biosynthesis of chlorophyll is accomplished by a series of catalysis. The glutamyl-tRNA reductase (GluTR)-catalyzed reduction of glutamyl-tRNA is the rate-limiting and a pivotal regulation step in the chlorophyll biosynthetic pathway. In chloroplast-containing photosynthetic organisms, GluTR-binding protein (GluBP) is a recently identified spatial regulator that allocates GluTR for synthesis of different tetrapyrrole products. However, the underlying molecular mechanisms remain largely unclear.
Dr. Liu Lin and her colleagues from the Institute of Botany, Chinese Academy of Sciences (CAS), and the Institute of Biophysics, CAS, find that GluBP stimulates GluTR catalytic efficiency. The crystal structure of the GluTR-GluBP complex shows that GluBP binding promotes GluTR to a hydride-transferring state, revealing structural details for the catalytic process. These findings clarify a series of arguments regarding the activation and regulation of GluTR. The GluBP structure also suggests that GluBP may have a novel role in heme metabolism.
These results has been online published on Proceeding of National Academy of Sciences, April 21, 2014, and titled: “Crystal Structure of Arabidopsis Glutamyl-tRNA Reductase in Complex with Its Stimulator Protein”. PhD candidates Zhao Aiguo and Fang Ying are the co-first authors, and Dr. Liu Lin is the corresponding author.
This project was supported by National Natural Science Foundation of China, Ministry of Science and Technology, and CAS.

Structure of the GluTR-GluBP complex and a GluTR catalysis model. (A) The side view and top view of the GluTR-GluBP complex structure; (B) Surface potential of GluTR and the predicted tRNA-binding site; (C) Model of GluTR catalysis.
